Abstract
Introduction
Acquired hemophilia A (AHA) is a rare bleeding disorder in which acquired autoantibodies to endogenous Factor VIII (FVIII) decrease FVIII activity. AHA can lead to serious bleeding, with effective treatment requiring both immunosuppressive therapy (IST) and bypassing agents such as recombinant activated Factor VII (rFVIIa) or activated prothrombin complex concentrates (APCC). Disadvantages to bypassing agents include the inability to monitor response with standard lab assays, inconsistent hemostatic efficacy, and thrombosis (Astermark et al. 2007).
Recombinant porcine FVIII (rpFVIII) ), by virtue of its incomplete homology in the A2 and C2 domains of the FVIII molecule, has decreased reactivity with anti-human FVIII (hFVIII) inhibitors (Mulliez et al. 2014), enabling its use in AHA. It also has the advantage of laboratory monitoring of FVIII activity levels and hemostatic efficacy after failure of bypassing agents (Trautmann-Grill et al. 2018).
Between July 2015 and April 2018, 14 patients with AHA were treated with rpFVIII at the University of North Carolina. Data from our cohort show that the initial anti-porcine Bethesda titer (pBIA) does not reliably predict the clinical response to rpFVIII treatment and is not correlated with the anti-human Bethesda titer (hBIA). We also present data showing lower total rpFVIII use for initial bleed resolution when rpVIII is used up front, as compared to use as rescue therapy. Lastly, we validated our dosing algorithm which uses much lower than FDA-recommended doses with 10 more patients than in our previously reported patient series (Martin et al. 2016).
Methods
We analyzed clinical, pharmacy, and laboratory data from 14 patients treated with rpFVIII at the University of North Carolina for AHA from July 2015 to April 2018. All patients were initially treated according to our previously established dosing algorithm (Fig. 3). Investigational review board approval was obtained for our data collection and analysis.
Results
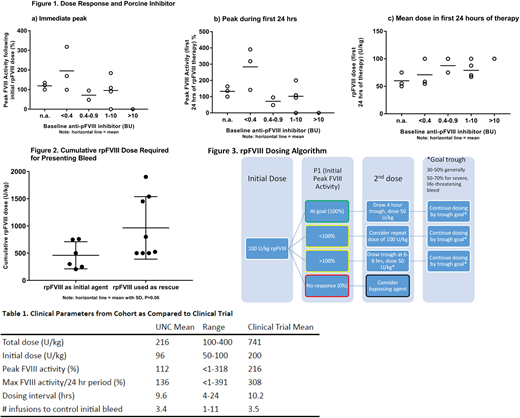
Of our 14 patients, 11 had a pBIA drawn prior to receiving rpFVIII, with 9/11 having a detectable porcine inhibitor, and only 2 of these showing no clinical response to rpFVIII infusion. There was no correlation between initial pBIA and FVIII response to rpFVIII infusion (Figs 1a-c). Only 2 patients lost their FVIII response and needed subsequent bypass agent use. One had a peak pBIA of 212 BU, and the other had a pBIA of 7, though 3 other patients who developed high titer pBIA did not lose their response. pBIA and hBIA showed poor correlation (r = 0.4, P = 0.2). 2/11 patients with available values had pBIA of <0.4, but had high titer hBIA.
13/14 patients achieved excellent resolution of their presenting bleed at much lower than FDA-recommended doses (see Fig 1c and Table 1). In 12/14 patients, the inhibitor resolved after IST. rpFVIII was given as up front therapy in 6/14 patients (i.e., preceded by < 24 hours of bypassing agent), and they required less cumulative rpFVIII for resolution of the presenting bleed compared to the 8 patients given rpFVIII as rescue therapy (up front mean dose = 400 U/kg, rescue mean dose = 663 U/kg, P=0.06, see Fig 2).
Conclusion
Our data validate our previously established dosing algorithm, providing hemostatic efficacy at lower than FDA-approved doses, and allowing for rapid and standardized dosing adjustments based on laboratory and clinical response without delaying initial treatment. As the initial pBIA does not reliably predict response to rpFVIII, and is not correlated with the hBIA, our practice has been to initiate rpFVIII therapy without waiting for results of the pBIA. The pBIA can be useful in case of repeat bleeding events, though not in a predictable manner. Our data also suggest lower dose requirements when used up front, rather than as rescue therapy, which can also allow pharmacy cost savings.
Key:UniQure BV: Research Funding. Ma:CSL: Consultancy; Shire: Honoraria, Research Funding; Novo Nordisk: Consultancy; CVS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal